Why calibrate?
The use of calibration for any metrological technique is critical to confirm that measurements are reliable and reproducible. Flow cytometry measurements are focused around the quantitation of light, in the form of light scatter and fluorescence. Flow cytometry data is displayed in arbitrary units which cannot be directly compared between instrument settings or different instruments without first being converted to known (calibrated) units (Figure 1A, B).
The calibration of flow cytometer light scatter and fluorescence are both possible, and commercially available reagents and software are available. While not heavily utilized by the cellular analysis field, for whom most flow cytometers are predominantly designed for, the use of calibration is essential for the small particle field where sensitivity is limited and no flow cytometer is currently capable of detecting the full range of extracellular vesicles.
The calibration of data not only allow instrument to instrument comparisons but can also enable better characterization of the particles of interest e.g. deriving size or the number of copies of protein on their surface (Figure 1E, F).
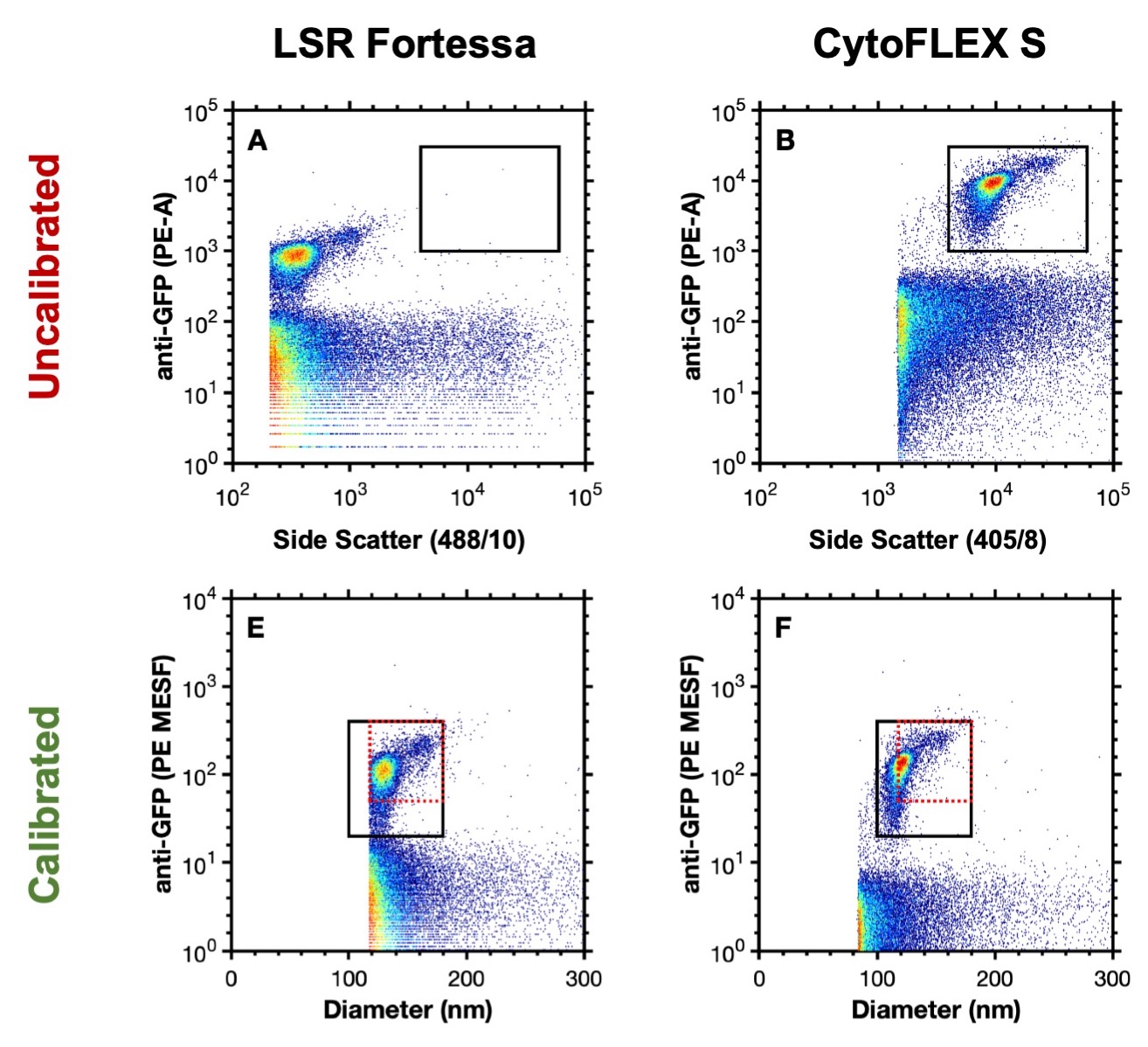
Figure 1. Uncalibrated data analyzing murine leukaemia virus (124 nm) on an LSR Fortessa (A) and CytoFLEX S (B). Calibrated data analyzing murine leukaemia virus on an LSR Fortessa (E) and CytoFLEX S (F). Data from Welsh J A, Jones J.C. Tang V A., Fluorescence and light scatter calibration allow comparisons of small particle data in standard units across different flow cytometry platforms and detector settings., Cytometry Part A, doi: 10.1002/cyto.a.24029.